FRANCE: Order as regards the experimental use of pilotless aircrafts for pesticides spraying
23 October 2019
FRANCE: Withdrawal of chlorothalonil-based products
12 November 2019In 2018, the Ministries of Agriculture and Ecological Transition asked Anses to make recommendations aimed at strengthening the regulatory framework for the protection of bees and other pollinators in France. Following this request and the first issued recommendations (Referral No. 2018-SA-0147 – in French), Anses began working on an update of the risk assessment procedure for bees and other pollinators in the context of Plant Protection Products authorisations (PPPs). On 28 October 2019, Anses published the conclusions of this work and its recommendations (Referral No. 2019-SA-0097 – in French).
Anses recommends replacing the current bee risk assessment scheme (based on SANCO/10329, 2002 and EPPO, 2010), by an approach based on the current version of the EFSA Bee guidance document (version of 4 July 2014, EFSA Journal 2013;11(7):3295), pending an harmonised approach is validated at European level. It is noted that the EFSA guidance document (2013) has not been adopted at EU level and is currently under revision by EFSA subsequent to a mandate from the European Commission (see our previous articles on this topic).
Anses recommendations concerning the new risk assessment scheme for bees and other pollinators are detailed below.
Effect assessment
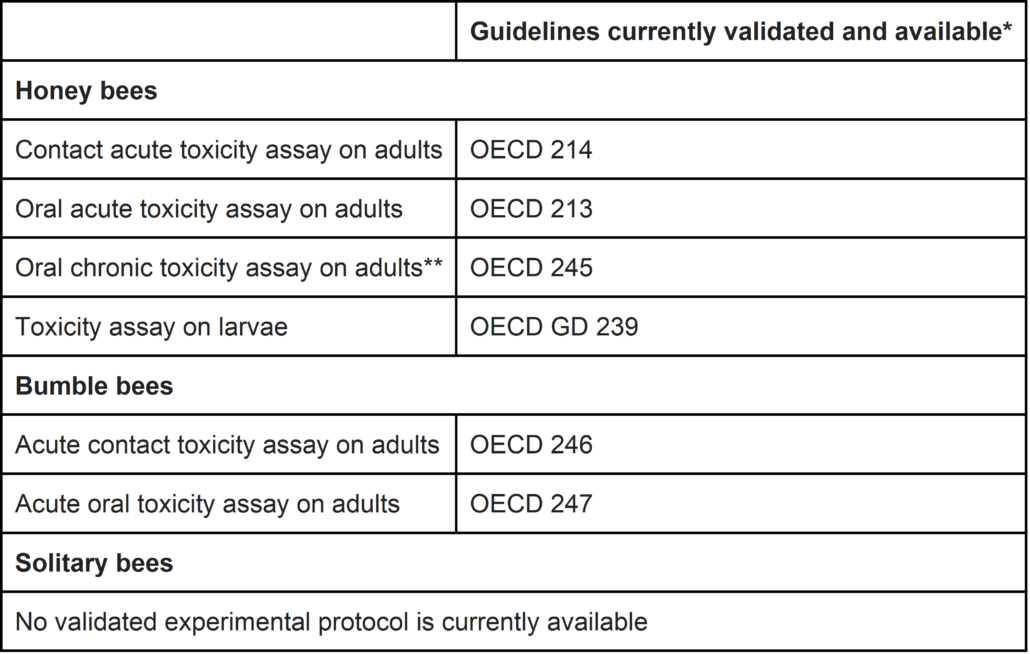
Studies conducted with PPPs are recommended regardless of the type of PPP and regardless of the number of active substances they contain. Studies on honey bees and bumble bees are currently recommended.
Recommended laboratory toxicity studies
* on 14 June 2019
** Study required for all PPPs as soon as a chronic exposure to PPP cannot be excluded for any of the EFSA exposure scenario.
Recommended semi-field (tunnel) and field studies
Honey bees
When an acceptable risk cannot be demonstrated with laboratory studies, the recommended higher tier studies are those referred to in the European Commission documents in relation to Regulations (EU) No. 283/2013 and 284/2013:
- Oomen method (Oomen et al., 1992), for assessing sublethal effects of active substances and PPPs;
- OECD method No. 75 (ENV/JM/MONO(2007)22), for assessing sublethal effects of PPPs.
Anses also refers to recommendations for tunnel and field studies from the EPPO Standard PP1/170 (4) (EPPO, 2010).
Tunnel and field studies are assessed in light of the protection goal for bees: it must be demonstrated that “under field conditions there are no unacceptable effects on honeybee larvae, honeybee behaviour, or colony survival and development after use of the plant protection product in accordance with the proposed conditions of use” (Reg. (EU) No. 546/2011).
Other pollinators
Given the lack of validated standard protocol, no particular test is recommended by Anses. Anses however indicates that the studies should allow the assessment of an expected effect on the colony survival and on the colony ability to produce new fertile individuals that will perpetuate the cycle the following year.
Considered routes of exposure and risk assessments
With some exceptions, Anses recommends that exposure assessments be based on the EFSA Guidance Document (2013) for sprayed PPPs or for PPPs used for granular application or for seed treatment.
Initial risk assessments
For honey bees, it is noted that the scenario of oral exposure via guttation water is currently not recommended by Anses because the EFSA’s assessment method is deemed to be affected by too important uncertainties. In addition, guttation water is not regarded as a major exposure route. Exposure via guttation water may be considered once the methodology has been updated by EFSA.
A risk assessment for effects on the hypopharyngeal glands or for cumulative effects is also not recommended at this time due to the lack of validated experimental protocols available.
For bumble bees, only the acute risk for adults is considered. No further evaluation is recommended at this time due to the lack of validated experimental protocols available.
Regarding solitary bees, no assessment is recommended at this time due to the lack of validated experimental protocols available.
Risk quotients (“HQ”, “ETR”) should be compared to the threshold values as defined in the revision SANTE/10094/2015 (in the course of adoption) of the Regulation (EU) No. 546/2011. This revision will update the threshold values for the honey bee acute risk assessments on the basis of the threshold values defined in the current EFSA guidance document (2013). It is noted that document SANTE/10094/2015 does not mention threshold values for the honey bee chronic and larvae risk assessments, and for the bumble bee acute risk assessments. Nevertheless, in the absence of established threshold values, Anses recommends the use of the threshold values defined in EFSA (2013).
Refined risk assessments
When an acceptable risk cannot be demonstrated with laboratory studies, several options may be proposed:
- Mitigation measures for problematic scenario (i.e. different variants of the SPe 8 phrase and mitigation measures as reported in EFSA guidance document (2013)).
- Specific studies supporting the non relevance of certain exposure scenario or studies allowing to refine default exposure levels (residue studies conducted in pollen/nectar, residue dissipation studies, determination of the nectar sugar content of crops, etc.).
- Higher tier studies (e.g. tunnel or field studies, studies assessing the ability of foragers to return to the hive).
Assessments for microorganism-based PPPs
For microorganism-based PPPs, the same methodology as for chemical-based PPPs is recommended. Adaptations of available experimental protocols are however recommended to justify the lack of pathogenicity/infectivity of microorganisms.
Perspective of the recommended risk assessment scheme
Anses indicates that the recommendations are made on the basis of the current version of the EFSA guidance document (2013) and on the basis of validated experimental guidelines currently available. Therefore, Anses call for:
- An update of these recommendations after the revision of the EFSA guidance document (expected for March 2021).
- An update of these recommendations as soon as a new experimental protocol is validated (e.g. acute toxicity assay on adults – solitary bees, chronic toxicity assay on adults and toxicity assay on larvae – bumble and solitary bees).
- The development of new standardised assays for assessing the effects of microorganism-based PPPs.
- The determination of regulatory threshold values for the chronic risk assessment for adults and for the risk assessment for larvae.
To download (in French):
AVIS de l’Anses relatif à l’Evolution de la méthodologie d’évaluation du risque vis-à-vis des abeilles domestiques et des insectes pollinisateurs sauvages dans le cadre des dossiers de demande d’AMM des produits phytopharmaceutiques (Saisine n° 2019-SA-0097)
See also our previous articles:
EUROPE-EFSA: Workplan for the Revision of the 2013 Bee Guidance Published
FRANCE – ANSES: recommendations to strengthen the protection of bees
Lynxee consulting’s team is at your disposal to answer your questions.
Contact us! https://lynxee.consulting/en/contact/